Answer: The molar mass of the vapor comes out to be 43.83 g/mol. This problem is solved by using ideal gas equation. The ideal gas equation is shown below
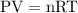
Step-by-step explanation:
Volume of gas = V = 247.3 mL
V = 0.2473 L
Pressure of gas = P = 745 mmHg
1 atm = 760 mmHg
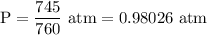
Temperature of gas = T = 100
 = 373 K
= 373 K
Given mass of gas = m = 0.347 g
Assuming molar mass of gas to be M g/mol
Assuming the gas to be an ideal gas, the ideal gas equation is shown below
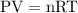
Here, n is the number of moles of gas and R is the universal gas constant.
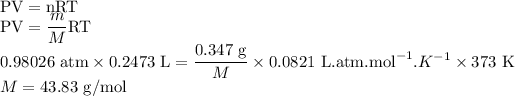
Hence, the molar mass of the vapor comes out to be 43.83 g/mol