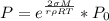Answer:
The vapor pressure for a mist is
Step-by-step explanation:
From the question we are given that
The radius is
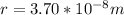
The temperature is
The vapor pressure of water
The density of water is
The surface tension of water is
Generally the equation of that is mathematically represented as
Where P is the vapor pressure for mist
R is the ideal gas constant = 8.31
making P the subject in the formula