Step-by-step explanation:
It is known that for high concentration of
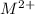 , reduction will take place. As, cathode has a positive charge and it will be placed on left hand side.
, reduction will take place. As, cathode has a positive charge and it will be placed on left hand side.
Now,
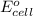 = 0 and the general reaction equation is as follows.
= 0 and the general reaction equation is as follows.
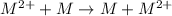
3.00 M n = 2 30 mM
E =
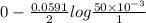
=
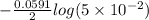
= 0.038 V
Therefore, we can conclude that voltage shown by the voltmeter is 0.038 V.