Answer:
V=3.10 L
Step-by-step explanation:
Considering the ideal gas equation as:-
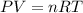
where,
P is the pressure = 1 atm
V is the volume = ?
n is the number of moles = 133 mmol = 0.133 mol
T is the temperature
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So,
T = (11.0 + 273.15) K = 284.15 K
R is Gas constant having value = 0.0821 L.atm/K.mol
So,
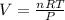
Now by putting the values in the above equation
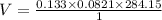
V=3.10 L
Therefore the volume of the gas will be 3.10 L.