Answer:
The value of the partial pressure of the oxygen
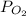 = 690 torr
= 690 torr
Step-by-step explanation:
Total pressure of the mixture of gases = 736 torr
The partial pressure of water vapor = 46 torr
From the law of pressure we know that
Total pressure = The partial pressure of water vapor + The partial pressure of oxygen

Put the values of pressures in above equation we get,
⇒ 736 = 46 +
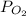
⇒
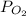 = 736 - 46
= 736 - 46
⇒
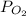 = 690 torr
= 690 torr
This is the value of the partial pressure of the oxygen.