The given reaction is incomplete. The complete reaction is as follows.
Ammonium phosphate
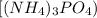 is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid
is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid
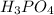 with liquid ammonia. Calculate the moles of phosphoric acid needed to produce 1.30 mol of ammonium phosphate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
with liquid ammonia. Calculate the moles of phosphoric acid needed to produce 1.30 mol of ammonium phosphate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
Step-by-step explanation:
Chemical equation for the given reaction is as follows.
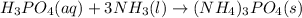
Therefore, moles of
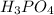 required will be calculated as follows.
required will be calculated as follows.
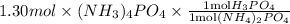
= 1.30 mol
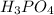
Therefore, we can conclude that the moles of phosphoric acid needed to produce 1.30 mol of ammonium phosphate are also 1.30 mol.