Answer:
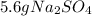
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
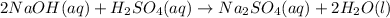
Therefore, since the masses of both of the reactants are given, one computes the available moles of sulfuric acid and those moles of it consumed by the sodium hydroxide as shown below:
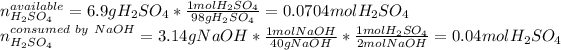
In such a way, since there is more available sulfuric acid than it that is consumed, the sodium hydroxide is the limiting reagent, consequently, the maximum mass of sodium sulfate turns out:
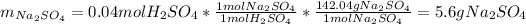
Best regards.