Answer:
123 J transfer into the gas
Step-by-step explanation:
Here we know that 123 J work is done by the gas on its surrounding
So here gas is doing work against external forces
Now for cyclic process we know that
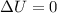
so from 1st law of thermodynamics we have
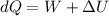
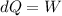
so work done is same as the heat supplied to the system
So correct answer is
123 J transfer into the gas