Answer:
0 J
Step-by-step explanation:
The work done by an ideal gas is given by the equation:
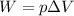
where
W is the work done by the gas
p is the pressure of the gas
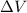 is the change in volume of the gas
is the change in volume of the gas
In this problem, we have a gas kept at a constant volume of
V = 2.00 L
This is an isochoric process (constant volume). Since the volume is constant, the change in volume is zero:

And therefore, this means that the work done by the gas is zero:
W = 0