Answer:
The answer to tnhe question is;
The weight percent of germanium to be added is 16.146 %.
Step-by-step explanation:
To solve the question, we note that the formula to calculate the weight percent of an element in terms of the number of atoms per cm³ in a 2 element alloy is given by,
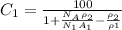
Where
N
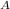 = Avogadro's Number
= Avogadro's Number
ρ₁ = Density of alloy whose weight percent is sought
ρ₂ = density of the other alloy
N₁ = Number of atoms per cubic centimeter
A₁ = Atomic weight of the element whose weight percent is sought
Therefore
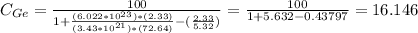
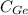 = 16.146 %.
= 16.146 %.