Answer:
0.00369 moles of HCl react with carbonate.
Step-by-step explanation:
Number of moles of HCl present initially =
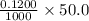 moles = 0.00600 moles
moles = 0.00600 moles
Neutralization reaction (back titration):

According to above equation, 1 mol of NaOH reacts with 1 mol of 1 mol of HCl.
So, excess number of moles of HCl present = number of NaOH added for back titration =
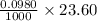 moles = 0.00231 moles
moles = 0.00231 moles
So, mole of HCl reacts with carbonate = (Number of moles of HCl present initially) - (excess number of moles of HCl present) = (0.00600 - 0.00231) moles = 0.00369 moles
Hence, 0.00369 moles of HCl react with carbonate.