Answer:
Amount of nitrogen consumed comes out to be 1050 g
Step-by-step explanation:
Number of moles of ammonia needed = 75 mole
The balanced reaction is shown below
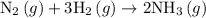
1 mole of ammonia is needed for producing 2 mole ammonia according to the balanced reaction.
Number of moles of nitrogen needed for producing 75 mole ammonia = 37.5 mole
Molar mass of nitrogen gas = 28 g/mole
Amount of nitrogen gas needed =
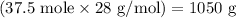
Amount of nitrogen gas needed = 1050 g