Answer: The theoretical potential of the given cell is -0.072 V
Step-by-step explanation:
The given chemical cell follows:
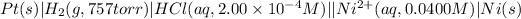
Oxidation half reaction:
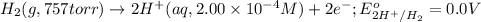
Reduction half reaction:
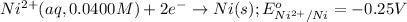
Net cell reaction:
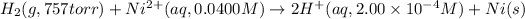
Oxidation reaction occurs at anode and reduction reaction occurs at cathode.
To calculate the
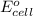 of the reaction, we use the equation:
of the reaction, we use the equation:
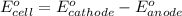
Putting values in above equation, we get:
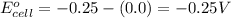
To calculate the EMF of the cell, we use the Nernst equation, which is:
![E_(cell)=E^o_(cell)-(0.059)/(n)\log ([H^(+)]^2)/([Ni^(2+)]* p_(H_2))](https://img.qammunity.org/2021/formulas/chemistry/college/vkrr9lkr6fb04voyoy4ki321ednm0o3ta5.png)
where,
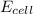 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V
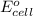 = standard electrode potential of the cell = -0.25 V
= standard electrode potential of the cell = -0.25 V
n = number of electrons exchanged = 2
![[H^(+)]=2.00* 10^(-4)M](https://img.qammunity.org/2021/formulas/chemistry/college/yz528fekdwgwlsvlnl9yn2w4lha25ixtbi.png)
![[Ni^(2+)]=0.0400M](https://img.qammunity.org/2021/formulas/chemistry/college/f5cl935ibhf8p99vf629svf9m50tscaas3.png)
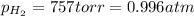 (Conversion factor: 1 atm = 760 torr)
(Conversion factor: 1 atm = 760 torr)
Putting values in above equation, we get:
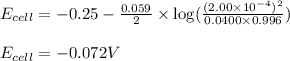
Hence, the theoretical potential of the given cell is -0.072 V