Answer:
8.60 g/cm³
Step-by-step explanation:
In the lattice structure of iron, there are two atoms per unit cell. So:
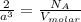 where
where
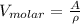 an and A is the atomic mass of iron.
an and A is the atomic mass of iron.
Therefore:
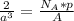
This implies that:
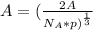
=
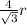
Assuming that there is no phase change gives:
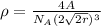
= 8.60 g/m³