Answer:
The volume of the gas at 716 mmHg comes out to be 40.43 mL. This problem is solved by using below equation.
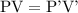 at constant temperature
at constant temperature
Step-by-step explanation:
Initial volume of gas = V = 37.5 mL
Initial pressure of gas = P = 772 mmHg
Final pressure of gas = P' = 716 mmHg
Assuming final volume of the gas to be V' mL.
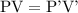 at constant temperature
at constant temperature
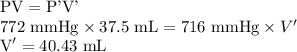
Hence, volume of oxygen gas at 716 mmHg is 40.43 mL.