Answer:
The answer to the question is;
1637.769 grams of water will need to be perspired in order to maintain his original temperature.
Step-by-step explanation:
Energy intake of the person = 4000 kJ
Energy required to vaporize 1 mole of water = 44.0 kJ
That is 44.0 kJ/mole
Therefore
The number of moles of water that can be vaporized by 4000 kJ is given by
(4000 kJ)/ (44.0 kJ/mole) = 90.91 moles.
Mass of one mole of water = Molar mass of water = 18.01528 g/mol
Since number of moles of water = (
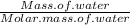 )
)
We therefore have
Mass of water = (Number of moles of water)× (Molar mass of water)
Mass of water = 90.91 moles× 18.01528 g/mol = 1637.769 g
The mass (in grams) of water that he would need to perspire in order to maintain his original temperature is 1637.769 g.