Step-by-step explanation:
For the given reaction equation, we will write the state of each specie as follows.
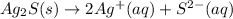
Since, silver sulfide (
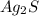 ) will remain in solid state. Therefore, it acts as a precipitate, that is, insoluble solid. Hence, it is insoluble.
) will remain in solid state. Therefore, it acts as a precipitate, that is, insoluble solid. Hence, it is insoluble.
And, expression for the equilibrium constant of this reaction is as follows.
![K_(eq) = ([Ag^(+)]^(2)[S^(2-)])/([AgS]^(2))](https://img.qammunity.org/2021/formulas/chemistry/college/n0jds4dlav7dim7lcp5ydrixa8q68m68wm.png)
For solids, it is considered to be equal to 1. Hence, the equilibrium constant expression will be as follows.
![K_(eq) = [Ag^(+)]^(2)[S^(2-)]](https://img.qammunity.org/2021/formulas/chemistry/college/uxommgtj9gkw914nnc5xkbtwbdhmnbty98.png)
Therefore, we can conclude that its equilibrium constant for this reaction will be greater than 1.