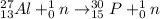Step-by-step explanation:
An alpha particles is basically a helium nucleus and it contains 2 protons and 2 neutrons.
Symbol of an alpha particle is
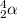 . Whereas a neutron is represented by a symbol
. Whereas a neutron is represented by a symbol
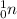 , that is, it has zero protons and only 1 neutron.
, that is, it has zero protons and only 1 neutron.
Therefore, reaction equation when an aluminum- nuclide transforms into a phosphorus- nuclide by absorbing an alpha particle and emitting a neutron is as follows.