Step-by-step explanation:
It is given that total energy is -443 kJ/mol and formula to calculate the lattice energy is as follows.
Total energy = heat of sublimation + bond dissociation energies + ionization energy for Cs + EA of
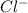 + lattice energy
+ lattice energy
-443 kJ/mol = 76 + 121 + 376 - 349 + Lattice energy
Lattice energy = (-443 - 76 -121 - 376 + 349) kJ
Lattice energy = -667 kJ
Therefore, we can conclude that -667 kJ is the magnitude of the lattice energy for CsCl.