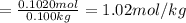Step-by-step explanation:
Mass of solute = 10.0 g
mass of solvent(water) = m
Volume of solvent( water) = v = 100.0 mL
Density of water= d =
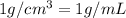
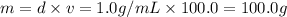
Mass of solution(M) = Mass of solute + mass of solvent
M = 10.0 g + 100.0 g = 110.0 g
Volume of the solution = V = 113 mL
Density of the solution = D
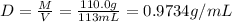
The density of the solution is 0.9734 g/ml.
Moles of phosphoric acid =
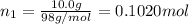
Moles of water =
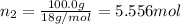
Mole fraction of phosphoric acid =
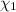
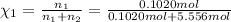
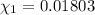
Mole fraction of water =
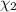
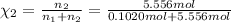
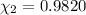
![[Molarity]=\frac{\text{Moles of solute}}{\text{Volume of solution(L)}}](https://img.qammunity.org/2021/formulas/chemistry/high-school/xr4p44e7md4zj1cp15ien0t9yodzdaqgj6.png)
Moles of phosphoric acid = 0.1020 mol
Volume of the solution = V = 113 mL = 0.113 L ( 1 mL = 0.001 L)
Molarity of the solution :
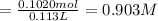
![[Molality]=\frac{\text{Moles of solute}}{\text{Mass of solvent(kg)}}](https://img.qammunity.org/2021/formulas/chemistry/high-school/fx9yqu702aw6h4pnzy8f7x3b9zt8x80915.png)
Moles of phosphoric acid = 0.1020 mol
Mass of solvent(water) = m =100.0 g = 0.100 kg ( 1 g = 0.001 kg)
Molality of the solution :