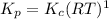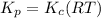This is an incomplete question, here is a complete question.
Consider the following chemical equilibrium:
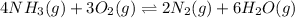
Now write an equation below that shows how to calculate Kp from Kc for this reaction at an absolute temperature T. You can assume T is comfortably above room temperature. If you include any common physical constants in your equation be sure you use their standard symbols, found in the ALEKS Calculator.
Answer :
The expression of
 will be,
will be,
![K_c=([N_2]^2[H_2O]^6)/([NH_3]^4[O_2]^3)](https://img.qammunity.org/2021/formulas/chemistry/college/is5k181jfzdy581prr0xx10xvwlwmrsmg9.png)
The expression of
 will be,
will be,
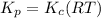
Explanation :
Equilibrium constant : It is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.
The equilibrium expression for the reaction is determined by multiplying the concentrations of products and divided by the concentrations of the reactants and each concentration is raised to the power that is equal to the coefficient in the balanced reaction.
As we know that the concentrations of pure solids and liquids are constant that is they do not change. Thus, they are not included in the equilibrium expression.
The given equilibrium reaction is,
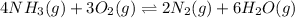
The expression of
 will be,
will be,
![K_c=([N_2]^2[H_2O]^6)/([NH_3]^4[O_2]^3)](https://img.qammunity.org/2021/formulas/chemistry/college/is5k181jfzdy581prr0xx10xvwlwmrsmg9.png)
The relation between
 and
and
 is:
is:
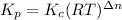
Form the above reaction we conclude that:
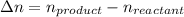
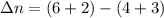
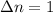
The expression of
 will be,
will be,