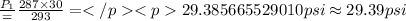Answer:
29.39 psi
Step-by-step explanation:
Assuming volume change then we can say
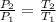 where P and T represent pressure and temperature in Kelvin respectively. Making
where P and T represent pressure and temperature in Kelvin respectively. Making
 the subject of the formula then
the subject of the formula then
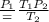
14 degrees celcius to kelvin will be 273+14=287 K, this is temperature 1
20 degrees celcius to Kelvin will be 273+20=293 K this is temperature 2
Pressure 2 is given as 30 psi