Step-by-step explanation:
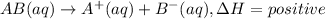
The reaction pertaining dissolution of ionic compound serves as system. Where as substance that is water in which an ionic compound is dissolved will act as surrounding.
According to question , on dissolving of an ionic compound in water makes the water colder which means that temperature of the water got lowered down due to dissolution of an ionic compound.From this we can conclude that heat is moving from surrounding to system , hence endothermic reaction.