Answer:
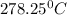
Step-by-step explanation:
In this case, Gay-Lussac's law is used to compute the required temperature, as it relates pressure and temperature, by knowing that the atmospheric standard temperature is 25 °C, as follows:
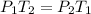
Thus, the initial pressure is known as 14.7 psi and the initial temperature 25 °C, which correspond to the atmospheric conditions. Nonetheless, the pressure into the cooker, should be:
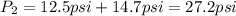
Since the 12.5 psi are above the atmospheric pressure. In such a way, the temperature inside the cooker turns out:
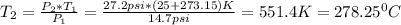
Best regards.