Step-by-step explanation:
As Iron (III) nitrate is an ionic compound so when it is dissolved in water then it will dissociate to give nitrate ions and ferric ions. Also, we know that like dissolves like and here iron (III) nitrate being an ionic compound will readily dissolve in water (polar solvent).
The chemical equation for this reaction is as follows.
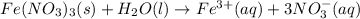
Hence, iron(III) nitrate considered soluble in water.
Now, equilibrium constant expression for this reaction is as follows.
![K_(eq) = [Fe^(3+)][(NO_(3))_(3)]^(3)](https://img.qammunity.org/2021/formulas/chemistry/college/o0x819m4r9vsy7veqe7brjxvmcax5m1vof.png)
Therefore, the equilibrium constant for this reaction will be greater than 1.