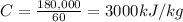Answer:
3000 kJ/kg
Step-by-step explanation:
The calorific value of a substance is the amount of heat produced per unit mass by the combustion of the substance.
It is given by:
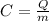
where
Q is the amount of heat released
m is the mass of the fuel
In this problem, we have:
m = 60 kg is the mass of fuel
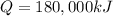 is the amount of heat released
is the amount of heat released
Therefore, the calorific value of the fuel is: