Answer:
2.42L
Step-by-step explanation:
Given parameters:
V₁ = 1.8L
T₁ = 293K
P₁ = 101.3kPa
P₂ = 67.6kPa
T₂ = 263K
Unknown:
V₂ = ?
Solution:
To solve this problem, we are going to use the combined gas law to find the final volume of the gas. The combined gas law expression combines the equation of Boyle's law, Charles's law and Avogadro's law;
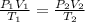
All the units are in the appropriate form. We just substitute and solve for the unknown;
101.3 x 1.8 / 293 = 67.6 x V₂ / 263
V₂ = 2.42L