Answer:
The number of moles of oxygen gas comes out to be 0.0548 mole
Step-by-step explanation:
Given volume of gas = V = 3.0 L
The mixture contains 30 % oxygen gas by mole.
Pressure of mixture of gas = P = 2.0 atm
Temperature = T = 400 K
Assuming n be the total number of moles of the mixture of gas.
The ideal gas equation is shown below

The mixture contains 30% oxygen gas by mole
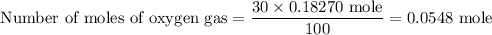
Number of moles of oxygen gas is 0.0548 mole