Answer:
The number of electrons in the outer energy level of oxygen is 6.
Step-by-step explanation:
Oxygen is present in the group 16 of the periodic table. The electronic configuration of group 16 element is
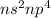 . The number of electrons in outer energy level of group 16 element is always found to be 6.
. The number of electrons in outer energy level of group 16 element is always found to be 6.
The addition of the number of the electrons present in the valence shell orbitals of oxygen will give the total number of electrons present in the outer energy level.
The number of electron in the outer energy level of oxygen is (2 + 4) = 6.