Answer:
The final pressure of the whole system is 34.80 atm.
Step-by-step explanation:
Given that,
Volume = 45.0 ml
Volume of first bulb = 77.0 mL
Pressure = 8.89 atm
Volume of second bulb = 250 mL
Pressure = 2.82 atm
Volume of third bulb = 21.0 mL
Pressure = 8.42 atm
We need to calculate the final pressure of the whole system
Using formula of pressure
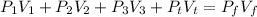
Where,
 = pressure of first bulb
= pressure of first bulb
 = pressure of second bulb
= pressure of second bulb
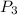 = pressure of third bulb
= pressure of third bulb
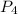 = initial pressure of tube
= initial pressure of tube
 = Volume of first bulb
= Volume of first bulb
 =Volume of second bulb
=Volume of second bulb
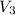 = Volume of third bulb
= Volume of third bulb
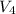 = Initial volume of tube
= Initial volume of tube
Put the value into the formula
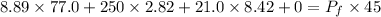
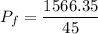
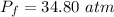
Hence, The final pressure of the whole system is 34.80 atm.