Answer:
e. 1.2 x 10²³
Step-by-step explanation:
According to the problem, The current equation is given by:
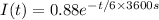
Here time is in seconds.
Consider at t=0 s the current starts to flow due to battery and the current stops when the time t tends to infinite.
The relation between current and number of charge carriers is:
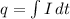
Here the limits of integration is from 0 to infinite. So,
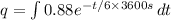
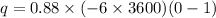
q = 1.90 x 10⁴ C
Consider N be the total number of charge carriers. So,
q = N e
Here e is electronic charge and its value is 1.69 x 10⁻¹⁹ C.
N = q/e
Substitute the suitable values in the above equation.
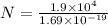
N = 1.2 x 10²³