Answer: Atomic number is reduced by two and mass number is reduced by four
Step-by-step explanation:
Radioactive decay of the nuclei happens when a nuclei has to attain some sort of stability. It undergoes various decay processes and releases various particles during these decay process.
One of decays is alpha decay in which the nucleus emits alpha particles. Alpha decay is radioactive decay in which a unstable atomic nucleus emits an alpha particle (helium nucleus) and transforms into an atom with an atomic number that is reduced by two and mass number that is reduced by four.
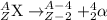
where Z = atomic number
A = mass number
X = symbol of element