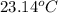Answer:
The final temperature when the lead and water reach thermal equilibrium is
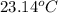
Step-by-step explanation:
Using the law of conservation of energy the heat lost by the lead ball is gained by the water.
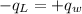 .............................1
.............................1
where
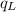 is the heat energy of the lead ball;
is the heat energy of the lead ball;
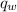 is the heat energy of water;
is the heat energy of water;
but q = msΔ
T...............................2
substituting expression in equation 2 into the equation 1;
Δ
T =Δ
T .............................3
where
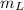 is the mass of the lead ball = 5.2 g
is the mass of the lead ball = 5.2 g
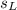 is the specific heat capacity of the lead ball = 0.128 Joules/g-deg
is the specific heat capacity of the lead ball = 0.128 Joules/g-deg
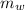 is the mass of water = volume x density = 34.5 ml x 1 g/ml = 34.5 g
is the mass of water = volume x density = 34.5 ml x 1 g/ml = 34.5 g
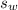 is the specific heat capacity of water = 4.184 J/g-deg
is the specific heat capacity of water = 4.184 J/g-deg
Δ
T is the temperature changes encountered by the lead ball and water respectively
inputting the values of the parameters into equation 3
5.2 g x 0.128 Joules/g-deg x (183-22.4) = 34.5 g x 4.184 J/g-deg x (
 -22.4)
-22.4)
 -22.4 =
-22.4 =
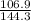
 -22.4 = 0.7408
-22.4 = 0.7408
 =
=
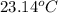
Therefore the final temperature when the lead and water reach thermal equilibrium is