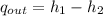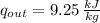Answer:
Step-by-step explanation:
First, it is required to find the absolute humidity of air at initial state:
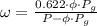
The saturation pressure at
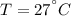 is:
is:
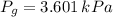
Then,
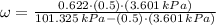
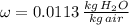
A simple cooling process implies a cooling process with constant absolute humidity. The specific entalphies for humid air are:
Initial state:
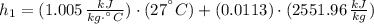
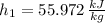
Final state:
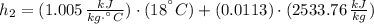
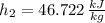
The specific energy that is removed is: