Answer:
The minimum concentration of urate would result in the precipitation is
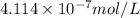 .
.
Step-by-step explanation:
Concentration of sodium ion in blood plasma =
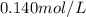
Concentration of urate ion in blood plasma =
![[(C_5H_3N_4)^-]](https://img.qammunity.org/2021/formulas/chemistry/college/n7knst7ytigosibhpk9nm6apwlhig80e8f.png)
The solubility product of sodium urate =
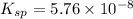
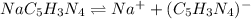
The expression of solubility product can be given as:
![K_(sp)=[Na^+][(C_5H_3N_4)^-]](https://img.qammunity.org/2021/formulas/chemistry/college/41d3pezhmtsixysjgmt5rrb7lg711my09k.png)
![K_(sp)=0.140 mol/L* [(C_5H_3N_4)^-]](https://img.qammunity.org/2021/formulas/chemistry/college/85repcpnyb73lyrlwz523c8rtjpnxsn397.png)
![5.76* 10^(-8)=[(C_5H_3N_4)^-]](https://img.qammunity.org/2021/formulas/chemistry/college/hiihu8t0pz722yhbzv3dygapbu0grpdjca.png)
![[(C_5H_3N_4)^-]=4.114* 10^(-7) mol/L](https://img.qammunity.org/2021/formulas/chemistry/college/7boxbgwzhuj8tw3yrw4zlr8a0d6qnwp4eb.png)
If the concentration of an urate ion in blood plasma increases more form
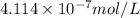 then the precipitation of sodium urate will take place.
then the precipitation of sodium urate will take place.