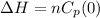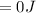Complete Question
The complete question is shown on the first uploaded image
Answer
a
As the valve is opened , the gas will flow into the empty container until the both containers have the same pressure
b
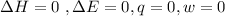
c
The driving force for this process is the increase in entropy this is because the movement of the internal energy of the gas into a larger volume, what this does is that it increases the amount of disorder(entropy).
Step-by-step explanation
In order to obtain the parameter in the part B of the question we are first obtain the initial pressure, using the ideal gas equation
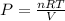
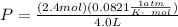
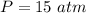
The next thing is to obtain the new pressure of the gas , using boyle's law
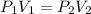
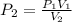
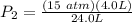
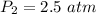
Since the this process is isothermal , the change in heat is equal to zero
i.e q = 0 J
The workdone to move the gas to the other container is zero because the the pressure at this second container is zero due to the fact that it is a vacuum
i.e
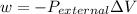
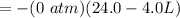
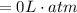
Since the change in heat is zero and the workdone is zero then the change in internal energy is equal to 0
This is because the change in internal energy is equal to a summation of change in heat and the workdone
i.e
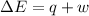
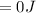
Generally the change in enthalpy is mathematically represented as
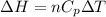
Since the temperature is zero this means that the change in temperature is zero , substituting this value for change in temperature into the equation for change in enthalpy