Answer: The initial concentration of weak acid solution is 0.243 M
Step-by-step explanation:
We are given:
pH of unknown acid at the halfway point of titration = 4.081
We know that:
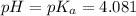 (At halfway point)
(At halfway point)
To calculate the
 , we use the equation:
, we use the equation:
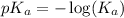
Putting values in above equation, we get:
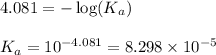
As, the initial pH of the acid is 2.348
- To calculate the hydrogen ion concentration, we use the equation:
![pH=-\log [H^+]](https://img.qammunity.org/2021/formulas/chemistry/high-school/rjo2yhb5oj9ry1fr4db1ujrazm6fh3vhke.png)
Putting values in above equation, we get:
![2.348=-\log [H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/azh5shm7bqirhhds2zjxsctrpjfo4fp0jg.png)
![[H^+]=10^(-2.348)=4.487* 10^(-3)](https://img.qammunity.org/2021/formulas/chemistry/college/p9lhzflyna3flxq73htdyp957s490pei13.png)
The chemical equation for the ionization of weak acid follows:
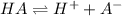
The expression of
 for above equation:
for above equation:
![K_a=([H^+][A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/cd33ttbdpkk5h562zi8dnbyn0akiej88j5.png)
As,
![[H^+]=[A^-]=4.487* 10^(-3)](https://img.qammunity.org/2021/formulas/chemistry/college/7wmzk3hvqn5krcao0kgaanbsnv78ex2bha.png)
Putting values in above equation, we get:
![8.298* 10^(-5)=((4.487* 10^(-3))* (4.487* 10^(-3)))/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/aghcus0lelq3lm8h0ntfn167te0v8g3dan.png)
![[HA]=0.243M](https://img.qammunity.org/2021/formulas/chemistry/college/n4tve6rlu0e7cvy8gaikpagtx2q6xk5iqt.png)
Hence. the initial concentration of weak acid solution is 0.243 M