Answer:
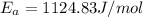
Step-by-step explanation:
Given that second order equation
K₁ = 0.0796 L/mol-s , T₁= 737⁰C
T₁ = 737 + 273 K = 1010 K
K₂ = 0.0815 L/mol-s , T₂=947°C
T₂=947+273 K= 1220 K
The activation energy given as follows
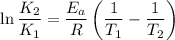
Now by putting the values we can get
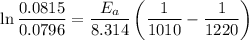
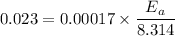
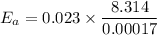
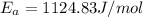
Therefore the activation energy will be 1124.83 J/mol