Answer:
See explanation.
Step-by-step explanation:
Hello,
For this exercise, it is convenient to assume a basis of 5 g of acetic acid which is the solute and 95 g of water which is the solvent, in such a way, one proceeds as follows:
(a) Molarity:
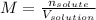
Thus:
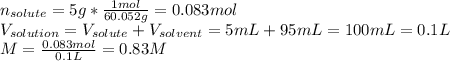
(b) Molality:
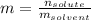
In this case, the moles were already computed and the mass of the solvent is in kilograms, thus:
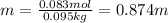
(c) Parts by mass:
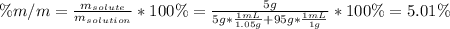
(d) Mole fraction:
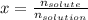
In this case, the moles of water are required:
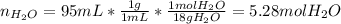
Since:
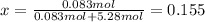
Best regards.