Answer:
The number of moles in Cesium Nitrate, CsNO3 is 2.2112 moles
Step-by-step explanation:
Moles of a substance n, is given by the mass, m of the substance divided by the molar mass, mm of the substance.
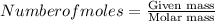
Molar mass of
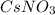 = a(Cs) + a(N) + a(O3)
= a(Cs) + a(N) + a(O3)
where a is the atomic mass;
a(Cs)=132.91 a(N)=14 a(O)=16
Therefore, Mm(CsNO3) = [132.91] + [14] + [16*3]
Mm(CsNO3) = 132.91+14+48
Mm(CsNO3) = 194.91
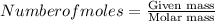
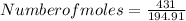
Number of moles = 2.2112moles
Hence, the number of moles in 431g of Cesium Nitrate is 2.2112moles