Answer:
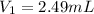
Step-by-step explanation:
Hello,
In this case, considering that the moles of hydrioiodic acid remain unchanged during the dilution process:
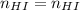
One could apply the following equality in terms of molarity:
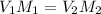
Whereas the subscript 1 accounts for the solution before the dilution and 2 after the dilution, therefore, the required volume of 6.00 M acid is:
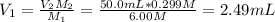
Best regards.