Answer:
 is dissociation constant and the value of
is dissociation constant and the value of
 is 4.98.
is 4.98.
Step-by-step explanation:
The pH of the solution = 2.95 M
![pH=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/fi7xbn2q6p6sosuqayohrecmxrbau6j4s5.png)
![2.95=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/high-school/mqg1gywgm2detykbh3b1uhsmt4p3kijr6i.png)
![[H^+]=10^(-2.95)=0.001122 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/mi8yklyzzx7r655ybg7dcu8qyacjx9zf69.png) ..[1]
..[1]
Concentration of unknown monoprotic acid = C = 0.1224 M
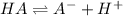
Initially
C 0 0
At equilibrium
(C-x) x x
The expression of a dissociation reaction can be written as;
![K_a=([A^-][H^+])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/high-school/n5xvssrqsuz68kas50gyuv3j3mnhrgjfp7.png)
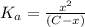
![[H^+]=x =0.001122 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/b756u0wo7nbhv62rc74czei8rz9ueg3fao.png) ( from [1])
( from [1])
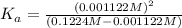
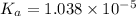
The value of
 :
:
![pK_a=-\log[K_a]](https://img.qammunity.org/2021/formulas/chemistry/college/ir8v80n39inyylpe73u5rdi9un2quhm56e.png)
![=-\log[1.038* 10^(-5)]=4.98](https://img.qammunity.org/2021/formulas/chemistry/high-school/dtjjfubpxrxeb6d42hzxy1fej5fypv42cx.png)
 is dissociation constant and the value of
is dissociation constant and the value of
 is 4.98.
is 4.98.