Answer:
The volume will decrease
Step-by-step explanation:
According to Boyle's Law:
"When a constant mass of an ideal gas is kept at constant temperature, the pressure of the gas is inversely proportional to its volume"
Mathematically:
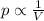
where:
p is the pressure of the gas
V is the volume of the gas
In this problem, we are asked what happens to the volume of a gas if its pressure increases. The equation above can be rewritten as
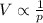
Therefore, we see that the volume is inversely proportional to the pressure: so, if the pressure increases, the volume will decrease.