43.56 grams of are produced if 16g of CH4 reacts with 64g of O2.
Step-by-step explanation:
Balance equation for the reaction:
CH4 + 2O2⇒ CO2 +2H2O
Data given : mass of CH4 =16 grams atomic mass = 16.04 grams/mole
mass of water 36 gram atomic mass = 18 grams/moles
mass of CO2=? atomic mass = 44.01 grams/mole
number of moles =
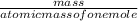 equation 1
equation 1
number of moles in CH4
n =
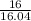
= 0.99 moles
Since combustion is done in presence of oxygen hence it is an excess reagent and methane is limiting reagent so production of CO2 depends on it.
From the equation
1 mole of CH4 gave 1 mole of CO2
O.99 moles of CH4 will give x moles of CO2
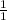 =
=
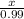
x = 0.99 moles of carbon dioxide
grams of CO2 = number of moles x atomic mass
= 0.99 x 44.01
= 43.56 grams of CO2 is produced.