Step-by-step explanation:
(1). It is known that in a reaction equation, reactants are placed or written on left hand side and products are written on the right hand side.
For example,
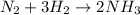
Hence, in a reaction equation you start with the reactants and end up with the products.
(2). The number of atoms in a reaction will remain the same because according to the law of conservation of mass, mass of reactants will be equal to the mass of products.
Therefore, number of atoms on the reactant side will be equal to the number of atoms on product side.