Answer: Option (A) is the correct answer.
Step-by-step explanation:
It is known that like dissolves like. So, water being a polar solvent is able to dissolve ionic compounds in it.
For example,
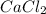 is an ionic compound as it's atoms contain a metal and a non-metal.
is an ionic compound as it's atoms contain a metal and a non-metal.
Therefore, the bond present present in it is an ionic bond. Hence, when it is dissolved in water then its reaction equation is as follows.

Thus, we can conclude that
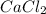 is soluble.
is soluble.