The question is incomplete, complete question is :
You need to produce a buffer solution that has a pH of 5.50. You already have a solution that contains 10 mmol (millimoles) of acetic acid. How many millimoles of acetate (the conjugate base of acetic acid) will you need to add to this solution? The pka of acetic acid is 4.74.
Answer:
33.11 millimoles of acetate we will need to add to this solution.
Step-by-step explanation:
To calculate the pH of acidic buffer, we use the equation given by Henderson Hasselbalch:
![pH=pK_a+\log(([salt])/([acid]))](https://img.qammunity.org/2021/formulas/biology/college/6usxe642bp3w274zbcv30her0kcessu95f.png)
Where :
tex]pK_a[/tex] = negative logarithm of acid dissociation constant of acid
[salt] = Concentration of salt
[Acid] = Concentration of salt
We have:
pH = 5.26
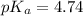
[salt] =
![[CH_3COO^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/lrrv43rj9xi2m5zj4tejvii4m8axiqs0kg.png) = ?
= ?
[acid] =
![[CH_3COOH]=10.0 mmol](https://img.qammunity.org/2021/formulas/chemistry/high-school/ad58jkvuyszqbqifavnz0fovyrxcolprrk.png)
![5.26=4.74+\log(([CH_3COO^-])/([10.0 mmol]))](https://img.qammunity.org/2021/formulas/chemistry/high-school/h74k0akw2erce67wy7z03r0fo9on4udnh7.png)
![[CH_3COO^-]=33.11 mmol](https://img.qammunity.org/2021/formulas/chemistry/high-school/seua6vuc50ha6xkurtkwgc51ziiqkiu1xb.png)
33.11 millimoles of acetate we will need to add to this solution.