Answer:
The velocity at the nozzle at inlet
 = 3584
= 3584
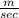
Step-by-step explanation:
Pressure at inlet
 = 1 ×
= 1 ×
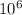 Pa
Pa
Temperature at inlet
 = 518 ° c = 791 K
= 518 ° c = 791 K
Mass flow rate =
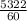
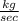 = 88.7
= 88.7
Gas constant for carbon die oxide is R = 189
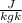
Mass flow rate inside the nozzle is given by the formula =
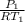 ×
×
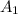 ×
×

⇒
 = = 1 ×
= = 1 ×
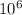 Pa
Pa
⇒ R
 = 791 × 189 = 149499
= 791 × 189 = 149499
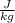
⇒
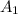 = 0.0037
= 0.0037
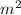
Put all the above values in above formula we get,
⇒ 88.7 =
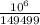 × 0.0037 ×
× 0.0037 ×

⇒
 = 3584
= 3584
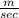
This is the velocity at the nozzle at inlet.