Answer: Average atomic mass of titanium on that planet is 46.52
Step-by-step explanation:
Mass of isotope Ti-46 = 45.95263
% abundance of isotope Ti-46 = 77.600 % =
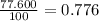
Mass of isotope Ti- 48= 47.94795
% abundance of isotope Ti-48 = 16.100%=
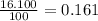
Mass of isotope Ti- 50 = 49.94479
% abundance of isotope Ti-50 = 6.300%=
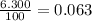
Formula used for average atomic mass of an element :
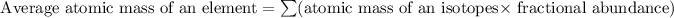
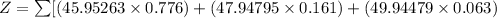
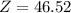
Average atomic mass of titanium on that planet is 46.52