1. 0.240 liters of water would be needed to dissolve 21.6 g of lithium nitrate to make a 1.3 M (molar) solution.
2. 2.9 M is the molarity of a solution made of 215.1 g of HCl is dissolved to make 2.0 L of solution.
3.83.3 ml of concentrated 18 M H2SO4 is needed to prepare 250.0 mL of a 6.0 M solution.
4. 135 ml of stock HBr will be required to dilute the solution.
5. 150 ml of water should be added to 50.0 mL of 12 M hydrochloric acid to make a 4.0 M solution
6. The pH of the resulting solution is 13.89
Step-by-step explanation:
The formula used in solving the problems is
number of moles=
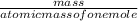 1st equation
1st equation
molarity =
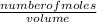 2nd equation
2nd equation
Dilution formula
M1V1 = M2V2 3rd equation
1. Data given
mass of Lithium nitrate = 21.6 grams
atomic mass of on emole lithium nitrate = 68.946 gram/mole
Molarity is given as 1.3 M
VOLUME=?
Calculate the number of moles using equation 1
n =
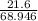
= 0.313 moles of lithium nitrate.
volume is calculated by applying equation 2.
volume =
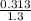
= 0.240 litres of water will be used.
2. Data given:
mass of HCl = 215.1 gram
atomic mass of HCl = 36.46 gram/mole
volume = 2 litres
molarity = ?
using equation 1 number of moles calculated
number of moles =
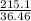
number of moles of HCl = 5.899 moles
molarity is calculated by using equation 2
M =
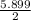
= 2.9 M is the molarity of the solution of 2 litre HCl.
3. data given:
molarity of H2SO4 = 18 M
Solution to be made 250 ml of 6 M
USING EQUATION 3
18 x V1= 250 x 6
V1 = 83.3 ml of concentrated 18 M H2SO4 will be required.
4. data given:
M1= 10M, V1 =?, M2= 3 ,V2= 450 ml
applying the equation 3
10 x VI = 3x 450
V1 = 135 ml of stock HBr will be required.
5. Data given:
V1 = 50 ml
M1= 12 M
V2=?
M2= 4
applying the equation 3
50 x 12 = 4 x v2
V2 = 150 ml.
6. data given:
HCl + NaOH ⇒ NaCl + H20
molarity of NaOH = 0.525 M
volume of NaOH = 25 ml
molarity of acid HCl= 75 ml
volume of HCl = 0.335 ml
pH=?
Number of moles of NaOH and HCl is calculated by using equation 1 and converting volume in litres
moles of NaOH = 0.0131
moles of HCl= 0.025 moles
The ratio of moles is 1:1 . To find the unreacted moles of acid and base which does not participated in neutralization so the difference of number of moles of acid minus number of moles of base is taken.
difference of moles = 0.0119 moles ( NaOH moles is more)
Molarity can be calculated by using equation 1 in (25 +75 ml) litre of solution
molarity =
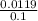
= 0.11 M (pOH Concentration)
14 = pH + pOH
pH = 14 - 0.11
pH = 13.89